Search This Supplers Products:Lithium BatterySodium BatterySodium Battery CellHome BatterySolar GeneratorRv Battery&Marine Battery
Ten Minutes To Learn About Sodium-Ion Batteries
sourceGoogle
publisherKayla He
time2024/02/28
- If you have no idea about sodium-ion batteries, you might as well take a look
Since the rise of areas such as electric vehicles and renewable energy, the demand for high-performance, cheap and sustainable energy storage has grown. In this field, sodium ion battery, as a high-profile technology, is gradually entering people's vision.
This blog will discuss the composition of sodium ion, the advantages and disadvantages of the battery, the technical route, the economy and other aspects, to take you to quickly understand this emerging energy storage technology.
Sodium Battery Technologysodium battery technology
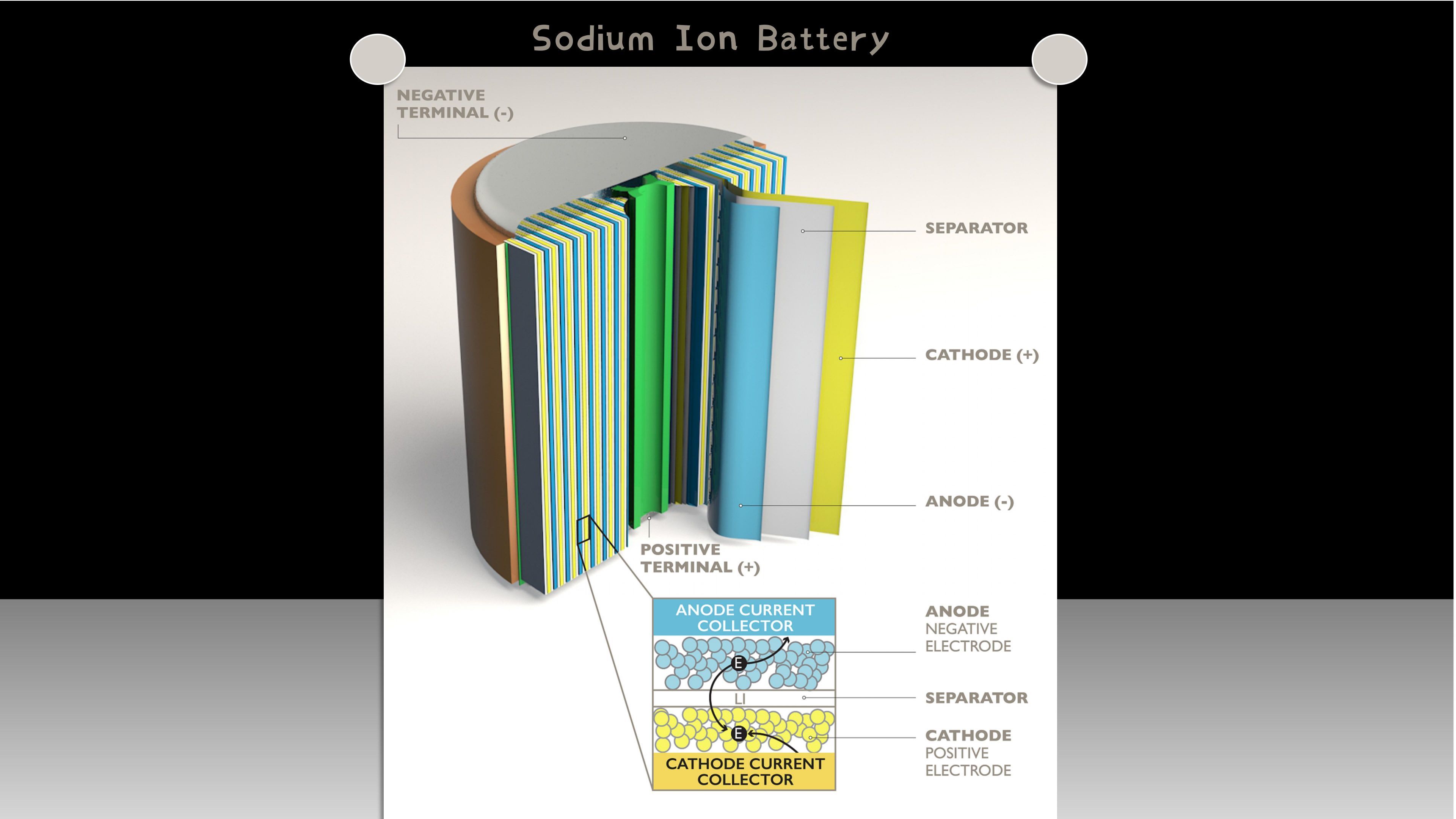
The composition of sodium-ion batteries
The basic composition of sodium-ion batteries is similar to that of lithium-ion batteries, including the positive electrode, negative electrode, electrolyte, and diaphragm. Among them, the positive electrode material of sodium ion usually uses oxide, phosphate or sulfide, while the negative electrode uses carbon material or alloy. Sodium ion battery is mainly composed of key components such as cathode material, cathode material, electrolyte and diaphragm. Sodium-ion batteries work in a similar way to lithium-ion batteries, and are "rocking chair". When charging, the sodium ion is removed from the cathode material and passes through the electrolyte embedded in the cathode material. At the same time, electrons move from the positive electrode through the external circuit to the negative electrode to maintain the charge balance of the whole system. The discharge process is the opposite of the charging process. The positive and negative material systems of sodium ion battery are the decisive factors, and the electrolyte is mainly selected and used with the positive and negative material systems.
Advantages and disadvantages of sodium-ion batteries

The advantages of sodium batteries over lithium-ion batteries are that:
1.Rich resources and low cost:Compared with the scarcity of lithium ions, sodium ions in the crust element energy storage more abundant, thus low cost, can be a good supplement to lithium ion battery, as of November 2022 data, sodium carbonate price for lithium carbonate price about 1 / 200, in addition to sodium battery anode adopt aluminum foil, can further reduce the cost
2.Wide temperature: all have a good capacity retention rate in the temperature range of-40℃ ~80℃
3.Good fast charging and ratio: the sodium ion battery electrolyte of the same concentration has higher ion conductivity than the lithium ion battery electrolyte, and the sodium ion has lower solvation energy in the polar solvent, making it have faster kinetic properties and higher conductivity in the electrolyte
4.Safety: Sodium battery can be stored and transported at zero voltage, without transportation safety risk, in short circuit, self-heating heat is less, no fire / explosion and other hidden dangers
5.Production: it has similar working principle and material composition with lithium ion battery, and the production experience and equipment can be partially compatible
The pain points of the practical application of sodium-ion battery technology are that:
The mass of sodium ion is heavier than that of lithium ion, and the electronegativity is less than that of lithium, so the energy density is less than that of lithium. The voltage of sodium ion battery is lower than that of lithium ion battery, so the sodium ion battery has lower specific capacity and low energy density.2) Sodium ions are larger, difficult to embed, and have poor cycle performance. The radius of sodium ion is larger than that of lithium ion, so the sodium ion is relatively stable in the rigid structure, which is difficult to reversible remove. Even if unembedding can occur, the dynamics of sodium ion embedding and detachment is very slow, and it can easily cause the structure of the electrode material to produce irreversible phase transition, thus reducing the cycle performance of the battery.
Technical route of sodium-ion battery
Positive electrode material
For the two pain points of sodium ion batteries, electrode materials are the key to improving their energy density, voltage and cycle performance. Only by developing the positive and negative electrode materials suitable for the stable removal of sodium ion, can the practical application of sodium ion battery be promoted. The existing cathode materials mainly include layered oxide materials, polyanionic materials, and Prussian blue / white materials. Among them, layered oxide material is the mainstream direction of sodium ion battery. Layer oxide is the fastest cathode material in development and is expected to be the first to achieve mass production.
Negative grade material
At present, the carbon-based materials that can be used as battery cathode materials mainly include graphite carbon materials and amorphous carbon (hard carbon and soft carbon) materials. Graphite materials, commonly used in the anode of lithium-ion batteries, cannot form stable compounds with sodium ions due to thermodynamic reasons, so it is difficult to use graphite as the anode material for sodium-ion batteries. Carbon nanomaterials mainly include graphene, carbon nanotubes, etc. Relying on surface adsorption to achieve sodium storage, it can achieve rapid charging and discharge, but the problems such as low efficiency and poor circulability make it difficult to obtain practical application. Amorphous carbon material with large layer spacing has become the most promising anode material for sodium ion batteries because of its high sodium storage capacity, low sodium storage potential and excellent cycle stability.
In carbon-based materials, compared with soft carbon materials such as graphite, hard carbon materials cannot be graphitized. The carbon layer arrangement of hard carbon material is less orderly than that of soft carbon material, and more micropores can be formed between the layers to facilitate the removal of sodium ions. Hard carbon material has many performance advantages, such as high sodium storage specific capacity, low sodium storage voltage, and good cycle performance. Meanwhile, it has the advantages of rich carbon source, low cost, non-toxic and environmental protection. Compared with graphite electrode, it also has more advantages in cold start and fast charging mode, and is the preferred sodium ion battery cathode material.
Electrolyte
The electrolyte of sodium-ion batteries is similar to the electrolyte of lithium-ion batteries, which can use part of the production equipment and technology of existing lithium-ion batteries. NaPF6 and NaClO4 are the two most frequently studied sodium salts. NaPF6 Until 300℃, with the highest conductivity in the PC based (propylene carbonate) electrolyte. Because its synthesis principle is similar to LiPF6, it can be compatible with the current lithium-ion battery manufacturing process and equipment in terms of manufacturing process, and has become the mainstream direction of sodium-ion battery electrolyte. NaClO4 It has the advantages of fast ion migration speed, strong thermal stability and low cost, but its insufficient high water content, easy explosion and high toxicity affect its practical application. Compared with the traditional sodium salts NaPF6 and NaClO4, sodium containing fluorosulfonyl groups (NaTFSI, NaFTFSI, NaFSI, etc.) has high thermal stability and non-toxicity, but because its anion is corrosive to aluminum foil fluid, it is rarely used as a separate sodium salt.
Economics of Sodium Ion Batteries
Features of Sodium Ion Battery
IFrom an economic point of view, sodium-ion batteries have obvious advantages over lithium-ion batteries, mainly reflected in the cost of raw materials and production costs. The wide distribution and low price of sodium resources provide strong support for the commercial application of sodium ion batteries. With the gradual implementation of the application scenarios around the development of sodium ion battery, it is expected that the sodium ion battery market will gradually expand, and become an indispensable part of the electrochemical market. According to the calculation of the brokerage, it is expected that in 2025, the cost of layered oxide system and polyanion system will be lower than the cost advantage of lithium battery.
Conclusion
With the acceleration of energy transformation and electrification, sodium ion batteries have a broad future in the application of electric vehicles, energy storage systems and renewable energy. It is expected that in the next few years, the sodium ion battery market will continue to expand and become an important part of the energy sector. We can see that as a new energy storage technology, it has huge potential and development space. With the continuous progress of technology and the gradual maturity of the market, sodium ion battery will become one of the important pillars in the future energy field.
Welcome To Our Discussion Group For E-lary Battery.
Be respectful, be constructive, stay on topic, support other commenters, and report bad behavior.
For more insights on sodium-ion batteries, please leave a comment